Abstract
Background: Acute lymphoblastic leukemia (ALL) is a malignant disorder with a long-term remission of less than 50% of adult patients and of nearly 80% of children. Relapsed and refractory (r/r) adult and childhood B-ALL patients, have significant unmet medical needs. Adoptive transfer of patient-derived T cells engineered to express a chimeric antigen receptor (CAR) by viral vectors has achieved complete remission and durable response in highly refractory populations (June CH et al. Science 2018). In addition, unmodified Cytokine Induced Killer (CIK) cells (CD3+, CD56+ T cells) have clearly demonstrated a high profile of safety in ALL patients (Introna M et al. Biol Blood Marrow Transplant. 2017). Here, we demonstrate the feasibility and reproducibility of a GMP-compliant clinical-grade culture and gene-modification protocol of allogeneic CIK cells using the non-viral Sleeping Beauty (SB) transposon system (Singh H et al, Plos One 2013) to obtain CD19CAR expressing CIK cells (Magnani CF et al, Oncotarget 2016, Magnani CF et al, Hum Gene Ther. 2018, Biondi A et al. J Autoimmun. 2017) starting from a limited amount of an easily available material such as peripheral blood (PB).
Methods: Fifty mL of PB were centrifuged on Ficoll gradient to obtain mononuclear cells (PBMCs). PBMCs were then simultaneously electro-transferred with the SB GMP-grade DNA transfer CD19.CAR/pTMNDU3 plasmid (human 3rd generation anti-CD19CD28OX40z CAR under the pTMNDU3 promoter), and transposase pCMV-SB11 plasmid (kindly provided by L. Cooper, MDACC, Houston, TX, USA). CIK populations (Introna M et al, Haematologica 2007) were then generated according to the method enclosed in the filed patent EP20140192371 (Magnani CF et al, Oncotarget 2016). The manufacturing process and the quality control tests were performed in a good manufacturing practices (GMP) academic cell factory authorized by Agenzia Italiana del Farmaco (AIFA) in the context of an ongoing phase I clinical trial (NCT03389035) for children and adults with relapsed/refractory B-cell precursor ALL post hematopoietic stem cell transplantation (HSCT).
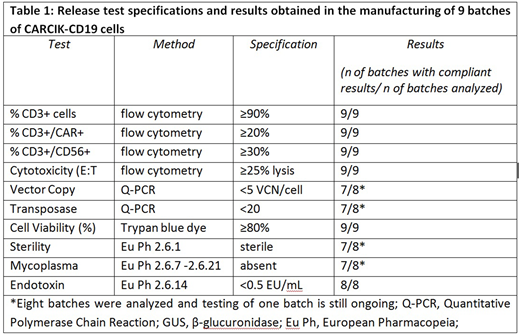
Results: We manufactured nine batches by seeding a mean of 102.52x106 allogeneicPBMCs derived from 50 ml of peripheral blood (range 46.1 - 193.17x106). After 21-22 days of culture the mean harvesting was 5.0x109 nucleated cells (range 1.15 - 16.00x109) with a mean viability of 97.56% (min. 95.24% - max 99.43%). These cells were mostly CD3+ lymphocytes (mean 98.54%, min. 94.85% - max 99.68%) which had a median fold increase of 87.3. Expanded CD3+ cells expressed CD56+ and surface CAR at variable levels among the batches (mean 44.79% and 43.78%, respectively) and had a median vector copy number (VCN) of 2.56 VCN/cells, according to pre-clinical data (Magnani CF et al, Hum Gene Ther. 2018). In all the nine batches, CARCIK-CD19 cells demonstrated potent and specific in vitro cytotoxicity towards the CD19+ REH target cell line (mean 82.96%, min. 61.89% - max 97.72%). Cell products appear to be highly polyclonal and no signs of genotoxicity by transposon insertions could be observed by integration site (IS) analysis performed by Sonication Linker Mediated (SLiM)-PCR and Illumina MiSeq sequencing. The GMP batches were released after about 10 days after the end of production. Quality control release specifications and results are reported in Table 1.
Conclusions: Overall, these results demonstrate that clinical-grade SB transduction of allogeneic CIK cells with CD19 CAR is feasible and allows rapid and efficient expansion of highly potent CARCIK-CD19 cells starting from easily available small amounts of PB, with important implications for non-viral technology. In summary our data represent a solid ground for the future development of further SB-based platforms. A clinical trial investigating allogeneic CARCIK-CD19 in r/r pediatric and adult ALL post HSCT is currently ongoing (NCT03389035).
Gritti:Autolus: Consultancy. Rambaldi:Celgene: Consultancy; Omeros: Consultancy; Novartis: Consultancy; Italfarmaco: Consultancy; Pfizer: Consultancy; Amgen Inc.: Consultancy; Roche: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal